Synthesis of Chiral Materials
Group Members: Brian Bloom, Zheni Georgieva, Yiyang Lu
Collaborators: Dali Sun (NC State University)
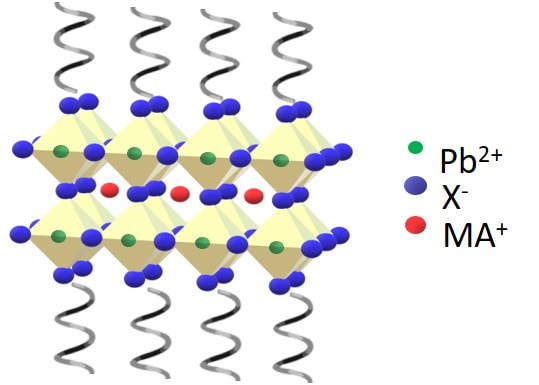
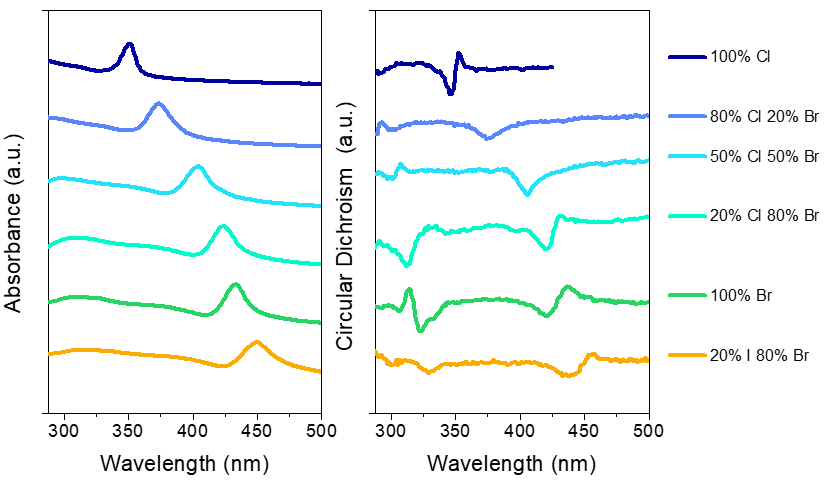
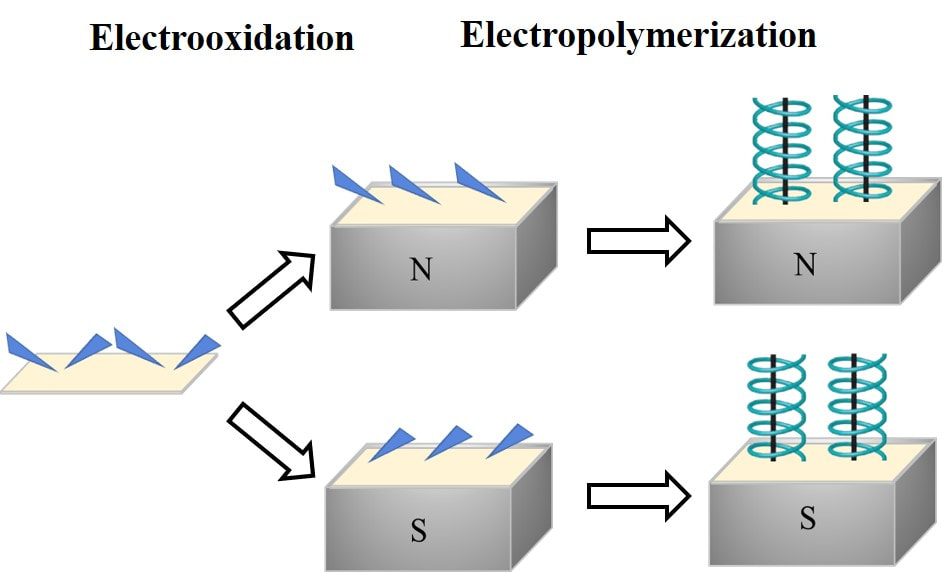
Chiral nanomaterials represent a new class of materials with promising properties for applications in the fields of optoelectronics and spintronics, amongst others. This part of our group is focused on the synthesis of new chiral nanomaterials and understanding mechanistically how the chirality manifests. These studies are pointing to structure – property relationships for the rational design of new chiral materials.
Collaborators: Dali Sun (NC State University)
Chiral nanomaterials represent a new class of materials with promising properties for applications in the fields of optoelectronics and spintronics, amongst others. This part of our group is focused on the synthesis of new chiral nanomaterials and understanding mechanistically how the chirality manifests. These studies are pointing to structure – property relationships for the rational design of new chiral materials.